Most organisms, including humans, exhibit daily rhythms in their behavior and physiology. In most cases, these rhythms are generated by endogenous processes referred to as circadian oscillators. These oscillators provide temporal structure to an organism's physiological processes. Nearly all functions of the ody show significant daily variations, including arousal, cognition, learning, memory, motor performance and perception. This temporal variation obviously plays an important role in the body's homeostatic mechanisms and has a major impact on the function of the nervous system.
Mammals have evolved a set of anatomically discrete cell populations that function as a physiological system to provide temporal organization on a circadian time scale. These structures are commonly referred to as the circadian system and can be localized to a pair of structures in the hypothalamus known as the suprachiasmatic nucleus or SCN. Importantly, when SCN cells are removed from the organism and maintained in a brain slice preparation, they continue to generate 24-hour rhythms in electrical activity, secretion, and gene expression. Previous studies suggest that the basic mechanism responsible for the generation of these rhythms is intrinsic to individual cells in the SCN.
In order to function adaptively, these cells must be synchronized to the exact 24 hr cycle of the physical world. The daily cycle of light and dark is the dominant cue used by organisms, including humans, to synchronize their biological clocks to the environment. Therefore, in the simplest case, a circadian system can be modeled as having three components: 1) an oscillator or clock responsible for the generation of the daily rhythm, 2) input pathways by which the environment and other components of the nervous system provide information to the oscillator, and 3) output pathways by which the oscillator provides temporal information to a wide range of physiological and behavioral control centers.
The long-term goal of our research program is to understand each of these three components at different levels of organization from systems to molecular. Our laboratory uses two strategies to approach this goal. In one, a systems-level analysis is carried out on the effects of genetic and pharmacological manipulations on behavioral rhythms driven by the circadian system. The other strategy examines the effects of these manipulations on the cellular/molecular activity of neural populations that make up this system.
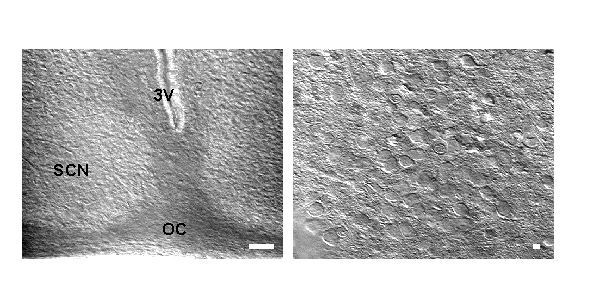
Live neurons in SCN brain slices visualized by IR DIC videomicroscopy. Left: image of SCN under lower power magnification. Bar = 100mM. 3V=third ventricle, OC=optic chiasm. Right: Higher power view of same slice. Bar = 10mm. This technology allows clear view of soma and, in some cases, processes of SCN cells. Rat, 14 days old.
Circadian disruption in models of neurodegeneration and aging.
A common complaint of patients suffering from neurodegenerative diseases such as Parkinson's and Huntington's Disease is the disruption of their sleep/wake cycle. It is not currently clear if this is caused by the underlying neurodegeneration, nor if circadian disruption can exacerbate the symptoms of PD and HD. We believe this is an extremely important factor in the quality of life of PD and HD patients, and have undertaken studies using models of both diseases to try to answer these questions.
Publications on neurodegeneration and aging:
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD. Age-Related Changes in the Circadian System Unmasked by Constant Conditions. eNeuro. 2015 Sep 22;2(4). pii: ENEURO.0064-15.2015. PMID: 26464996
- Kudo T, Loh DH, Tahara Y, Truong D, Hernández-Echeagaray E, Colwell CS. Circadian dysfunction in response to in vivo treatment with the mitochondrial toxin 3-nitropropionic acid. ASN Neuro. 2014 Jan 13;6(1):e00133. PMID: 24328694
- Loh DH, Kudo T, Truong D, Wu Y, Colwell CS. The Q175 mouse model of Huntington's disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One. 2013 Jul 30;8(7):e69993. PMID: 23936129
- Schroeder AM, Loh DH, Jordan MC, Roos KP, Colwell CS. Baroreceptor reflex dysfunction in the BACHD mouse model of Huntington's disease. PLoS Curr. 2011 Nov 4;3:RRN1266. PMID: 22069044.
- Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson's disease. Exp Neurol. 2011 Nov;232(1):66-75. Epub 2011 Aug 16. PMID: 21864527
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci. 2011 Jul 13;31(28):10201-5. PMID: 21752996
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS. Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease. Exp Neurol.2011 Mar;228(1):80-90. Epub 2010 Dec 22. PMID: 21184755.
Determination of the mechanisms that underlie the photic input to the SCN.
The SCN receives photic information directly through a monosynaptic projection from the retina known as the retinal hypothalamic tract (RHT). The RHT comprises of a distinct subset of retinal ganglion cells, which are directly light sensitive due to the presence of the photopigment melanopsin. These retinal ganglion cells utilize glutamate as a neurotransmitter. One of the fundamental features of circadian oscillators is that their response to environmental stimulation varies depending on the phase of the daily cycle when the stimuli are applied. For example, the same light treatment, which can produce phase shifts of the oscillator when applied during the night, has no effect when applied during the day. We have previously published data demonstrating that SCN neurons undergo a circadian oscillation in NMDA-evoked currents and calcium transients. We are currently exploring the mechanisms that underlie these state-dependent changes in excitatory response and have preliminary data indicating that the gene coding for the NR2B subunit is rhythmically regulated within the SCN.
We have become very interested in the possible role of BDNF as a signaling molecule responsible for the diurnal regulation of how SCN neurons respond to glutamate stimulation. BDNF is a neurotrophin that has been found to acutely modulate synaptic transmission. The receptors for BDNF, the TrkB tyrosine kinase, are expressed in the optic chiasm and SCN and levels of BDNF appear to be rhythmically secreted with peak expression during the night. Thus we have become interested in exploring the possible role of BDNF as a diurnal regulator of the NMDA receptor in the SCN. Our hypothesis is that BDNF acts both pre-synaptically to enhance the release of glutamate and post-synaptically to enhance the NMDA receptor mediated component of the SCN neurons to glutamate. We speculate that BDNF is producing the enhancement of NMDA currents through the regulation of the NR2B subunit. The combination of pre- and postsynaptic regulation would have the functional effect of increasing the coupling between the photic environment and the SCN during the night.
The retinal ganglion cells that make up the RHT also appear to express and utilize the neuropeptide pituitary adenylyl cyclase activating peptide (PACAP) as a transmitter to communicate with the SCN. In order to investigate the functions of PACAP in vivo, we collaborated with the Waschek laboratory to develop a novel mouse model in which the PACAP gene was disrupted by targeted homologous recombination. We have been exploring the behavioral consequences of the loss of PACAP for the light-response of the circadian system. Speculating that PACAP may function to modulate how SCN neurons respond to glutamate, we have been using electrophysiological and calcium imaging tools to examine possible cellular interactions between these co-transmitters.
Publications on mechanisms of photic entrainment:
- Vosko A, van Diepen HC, Kuljis D, Chiu AM, Heyer D, Terra H, Carpenter E, Michel S, Meijer JH, Colwell CS. Role of vasoactive intestinal peptide in the light input to the circadian system. Eur J Neurosci. 2015 Jul;42(2):1839-48. PMID: 25885685
- Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, WaschekJA, Colwell CS. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2010 Mar;31(5):864-75. Epub 2010 Feb 17. PMID: 20180841.
- Wang LM, Schroeder A, Loh D, Smith D, Lin K, Han JH, Michel S, Hummer DL, Ehlen JC, Albers HE, Colwell CS. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur J Neurosci. 2008 Apr;27(7):1771-9. PMID: 18380671
- Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci. 2006 Feb 16;7:15. PMID: 16483357
- Michel S, Clark JP, Ding JM, Colwell CS. Brain-derived neurotrophic factor and neurotrophin receptors modulate glutamate-induced phase shifts of the suprachiasmatic nucleus. Eur J Neurosci. 2006 Aug;24(4):1109-16. PMID: 16930436
- Kim YI, Choi HJ, Colwell CS. Brain-derived neurotrophic factor regulation of N-methyl-D-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons. J Neurosci Res. 2006 Nov 15;84(7):1512-20. PMID: 16983663
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004 Nov;287(5):R1194-201. PMID: 15217792
- Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci. 2001 Apr;13(7):1420-8. PMID: 11298803
Determination of the ionic mechanisms that underlie the generation of circadian rhythms in firing rate of SCN neurons.
Using whole cell patch recordings in a brain slice preparation, we have recently found that the magnitude of fast delayed rectifier potassium currents exhibits a diurnal rhythm that peaks during the day. Importantly, this rhythm continues in tissue from mice maintained in constant darkness, providing the first demonstration of the circadian regulation of an intrinsic voltage-gated current in mammalian cells. Blocking this current prevented the daily rhythm in firing rate in SCN neurons as measured with perforated patch current clamp recordings. We conclude that the fast delayed rectifier is necessary for the circadian modulation of electrical activity in SCN neurons, and represents an important part of the ionic basis for the generation of rhythmic outputs from these pacemaker cells. The mechanisms underlying the daily rhythm in fast DR currents are not known. A variety of evidence suggests that the Kv3.1 and 3.2 genes contribute to the channels that underlie the fast DR currents. Our immunocytochemical evidence clearly indicates the presence of the Kv3.1b and Kv3.2 channels in the SCN. It is possible that these genes are rhythmically transcribed in SCN neurons as the promotor region of the Kv3.1 gene contains both CRE and AP-1 sites. We believe that we have identified one of the key currents that are responsible for driving the circadian rhythms in membrane potential that are characteristic of SCN neurons.
Publications on ionic mechanisms underlying SCN firing rhythms:
- Kudo T, Block GD, Colwell CS. The Circadian Clock Gene Period1 Connects the Molecular Clock to Neural Activity in the Suprachiasmatic Nucleus. ASN Neuro 2015 Nov 9;7(6). pii: 1759091415610761. PMID: 26553726
- Kim YS, Kim YB, Kim WB, Yoon BE, Shen FY, Lee SW, Soong TW, Han HC, Colwell CS, Lee CJ, Kim YI. Histamine resets the circadian clock in the suprachiasmatic nucleus through the H1R-CaV 1.3-RyR pathway in the mouse. Eur J Neurosci. 2015 Oct;42(7):2467-77. PMID: 26215659
- Kudo T, Loh DH, Kuljis DK, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci 2011 Feb 23;31(8):2746-55. PMID: 21414897
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of A-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010 Feb;103(2):632-40. Epub 2009 Nov 25. PMID: 19939959.
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005 May;8(5):650-6. PMID: 15852012
Determination of the role of the neuropeptide VIP in the regulation of cellular communication within the SCN.
The mechanisms by which SCN neurons are coupled to each other are not yet known, but it is widely accepted that most SCN neurons express the neurotransmitter gamma-animobutyric acid (GABA), as well as the neuropeptide vasoactive intestinal peptide (VIP). In order to investigate the functions of VIP, we again collaborated with the Waschek laboratory to develop a new mouse model in which the VIP gene was disrupted by homologous recombination. We have been exploring the behavioral consequences of the loss of VIP for the generation of circadian rhythms on both behavioral and cellular levels. Electrophysiological analysis indicates that VIP enhances inhibitory synaptic transmission within the SCN. Together our work suggests that VIP is critically involved in both the generation of circadian oscillations as well as the normal synchronization of these rhythms to light. We feel that these observations highlight the importance of GABA and VIP in communicating light information from retino-recipient cells to the rest of the SCN.
Publications on VIP regulation of circadian function:
- Vosko A, van Diepen HC, Kuljis D, Chiu AM, Heyer D, Terra H, Carpenter E, Michel S, Meijer JH, Colwell CS. Role of vasoactive intestinal peptide in the light input to the circadian system. Eur J Neurosci. 2015 Jul;42(2):1839-48. PMID: 25885685
- Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013 Sep;110(5):1097-106. PMID: 23741043
- Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011 Jun;26(3):200-9. PMID: 21628547
- Schroeder A, Loh DH, Jordan MC, Roos KP, Colwell CS. Circadian Regulation of Cardiovascular Function: a role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol. Epub 2010 Oct 15. 2010 PMID: 20952671.
- Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, Waschek JA, Colwell CS. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2010 Mar;31(5):864-75. Epub 2010 Feb 17. PMID: 20180841.
- Loh DH, Abad C, Colwell CS, Waschek JA. Vasoactive Intestinal Peptide Is Critical for Circadian Regulation of Glucocorticoids. Neuroendocrinology. 2008 Jun 19. [Epub ahead of print] PMID: 18562786
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007 Mar;97(3):2553-8. PMID: 17151217
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005 Apr;8(4):476-83. PMID: 15750589
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004 Jul;92(1):311-9. PMID: 14973316
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003 Sep;90(3):1589-97. PMID: 12966176
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003 Nov;285(5):R939-49. PMID: 12855416
Determination the role of clock genes and the circadian system in the regulation of learning and memory processes.
We have found that a cellular model of learning and memory, Long Term Potentiation (LTP), is regulated on a circadian time scale. We are currently exploring the mechanism underlying this temporal regulation. Importantly, we have found that there is a daily rhythm in the expression of one of the clock genes (Period2) in the pyramidal neurons within the hippocampus. Furthermore, the induction of LTP itself regulates expression of this clock gene. While this work is still in the early stages, our observations would suggest that the hippocampus may function as a peripheral oscillation containing the same transcriptional/translational feedback loops responsible for circadian oscillations in the SCN.
We found that there is a circadian rhythm in the ability of mice to learn a simple behavioral task - contextual fear conditioning. In addition, the animals' ability to recall the learned behavior as well as the extinction (forgetting) of the behavior also show circadian variation. Furthermore, we have found that acute disruption of the circadian cycle can have negative effects on recall of conditioned fear. We are currently exploring the mechanisms underlying this regulation, focusing our attention on the circadian regulation of gene expression in the hippocampus and amygdala.
Publications on circadian regulation of learning and memory:
- Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One 2010 Sep 2;5(9). pii: e12546. PMID: 20824058.
- Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O'Dell TJ, Colwell CS. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009 Jun 10;1(3). PMID: 19570032.
- Chaudhury D, Loh DH, Dragich JM, Hagopian A, Colwell CS. Select cognitive deficits in Vasoactive Intestinal Peptide deficient mice. BMC Neurosci. 2008 Jul 10;9(1):63. [Epub ahead of print] PMID: 18616823.
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005 Jun;20(3):225-36. PMID: 15851529
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005 Nov;22(9):2231-7. PMID: 16262661
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002 Jun 15;133(1):95-108. PMID: 12048177
